The Genesis of Severe Acute Respiratory (Syndrome) Disease or SARS (Coronavirus – COVID – 2 and 19)
Updated: May 24, 2020
Authors: Robert O Young DSc. PhD. and Dr. Galina Migalko MD, NMD
16390 Dia del Sol, Valley Center, California 92082
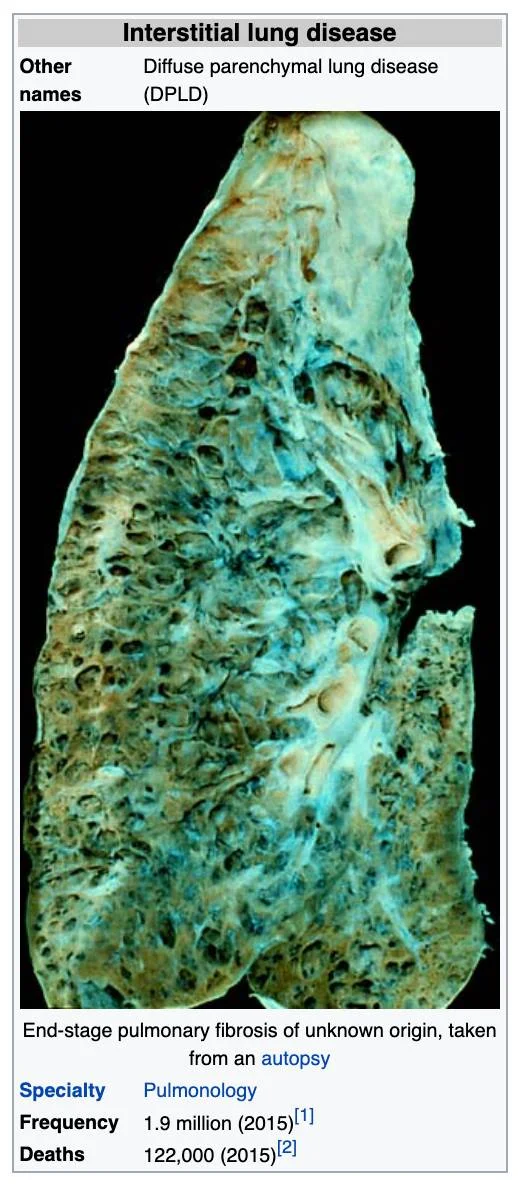
Interstitial Fluid Lung Disease (IFLD) of the Interstitium Organ The Cause and Self-Care to a Self-Cure for Lung Disease
Abstract
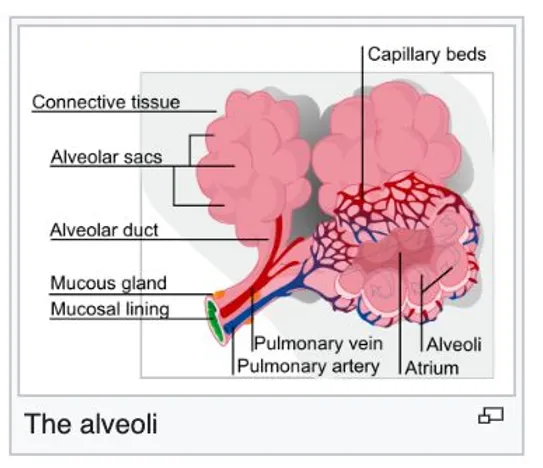
Interstitial lung disease (IFLD), or diffuse parenchymal lung disease (DPLD),[3] is a group of lung diseases affecting the Interstitium (the interstitial fluids or space around the alveoli (air sacs of the lungs).[4]
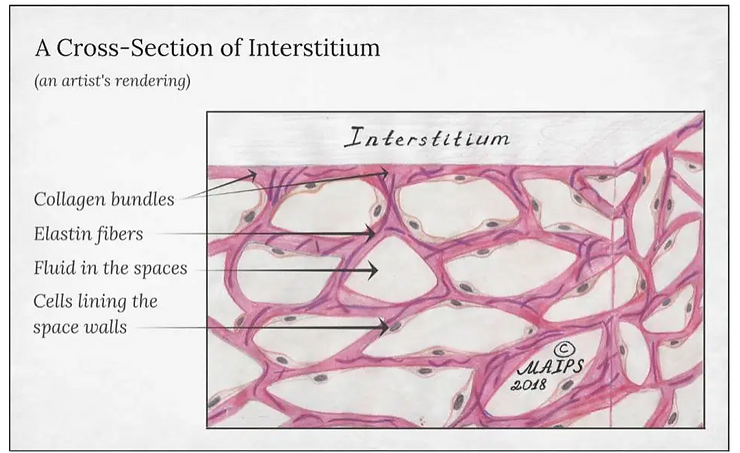
It concerns alveolar epithelium, pulmonary capillary endothelium, basement membrane, and perivascular and perilymphatic tissues. It occurs when metabolic, dietary, respiratory and environmental acids injure the lung tissues that triggers an abnormal healing response. Ordinarily, the body generates just the right amount of tissue to repair acid damage, but in interstitial lung disease, the repair process goes awry because of the acidic pH (ideal healing takes place at a pH of 7.365)[5]) of the interstitial fluids that effects the normal healing of the tissue around the air sacs (alveoli) and therefore becomes scarred anthickened.
This makes it more difficult for oxygen to pass into the bloodstream. The term ILD is used to distinguish these diseases from obstructive airways diseases but the cause of all lung disease is due to decompensated acidosis of the interstitial fluids (pH is below 7.2) that is systemic although affecting the weakest area of the lungs. These weaknesses can be attributed to lifestyle and dietary choices
There are specific types of interstitial lung disease in children. The acronym chILD is used for this group of diseases and is derived from the English name, Children’s Interstitial Lung Diseases – chILD.[6]
Prolonged interstitial lung disease may result in pulmonary fibrosis, but this is not always the case. Pulmonary fibrosis is specifically caused by the increase of acids in the interstitial fluids of the lung due to an inverted way of living, eating, drinking, breathing, thinking, feeling and believing. Interstitial lung disease of the Interstitium is associated with typical findings both radiographic (basal and pleural-based fibrosis with honeycombing) and pathologic (temporally and spatially heterogeneous fibrosis, histopathologic honeycombing, and fibroblastic foci).
Our findings using advanced technologies with non-invasive, non-radioactive 3D Bio-Electro Scanning of the Interstitial fluids of the largest organ of the body, the Interstitium to determine a complete chemistry, including pH. This testing has shown in all cases of interstitial fluid lung disease, including viral diseases, and malignancies with 98 percent accuracy for decompensated metabolic acidosis, of the IFLD, high levels of lactic acid and high levels of calcium. Since the interstitial fluids pass through every organ, gland and tissues we now have a complete picture of the functionality and chemistry of every organ, every gland and every tissue, including all bones and muscles.
Introduction
In 2015, interstitial lung disease, together with pulmonary sarcoidosis, affected 1.9 million people.[1] They resulted in 122,000 deaths.[2] and can be reversed with a specific alkaline lifestyle, including a specific alkaline diet, alkalizing exercise, alkalizing rectal infusion, alkalizing nebulization with specific water-based anti-oxidants, breathing techniques, transcendental meditation, specific alkalizing support with alkaline water, vitamins, minerals, herbal and the 12 tissue salt supplements and other techniques discovered by Robert O. Young PhD and Galina Migalko MD, NMD. [7]
The following are the names or specific symptoms given to Interstitial Lung Disease. The cause of ALL of these symptoms are the result of an acidic lifestyle, as listed below. [7]
The Symptoms of Interstitial Fluid Lung Disease (IFLD) of the Interstitium
The alveoli
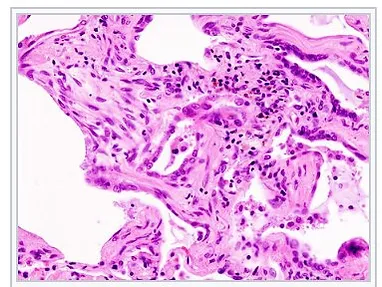
Micrograph of usual interstitial pneumonia (UIP). UIP is the most common pattern of interstitial pneumonia (a type of interstitial lung disease) and usually represents pulmonary fibrosis. H&E stain. Autopsy specimen.
ILD may be classified according to the symptoms of decompensated acidosis of the interstitial fluids of the lung.[8]
Methodology Classification by IFLD Symptoms is as follows:
Inhaled substances Inorganic:
Silicosis Asbestosis Berylliosis Industrial printing chemicals (eg. carbon black, ink mist)
Inhaled substances Organic:
Hypersensitivity pneumonitis
Drug-induced
Antibiotics Chemotherapeutic drugs
Antiarrhythmic agents
Connective tissue and Autoimmune diseases
Rheumatoid arthritis Systemic lupus erythematosus Systemic sclerosis Polymyositis Dermatomyositis Infection Outfection Atypical pneumonia Pneumocystis pneumonia (PCP)
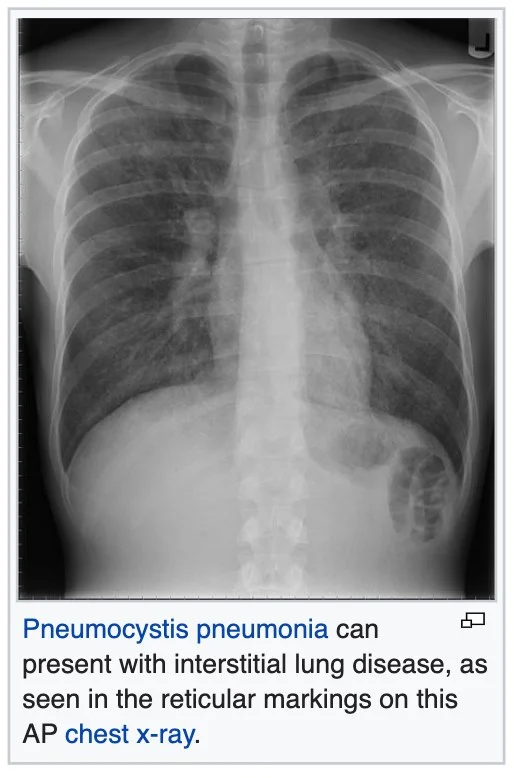
Tuberculosis Chlamydia trachomatis Respiratory Syncytial Virus Sarcoidosis Pulmonary fibrosis
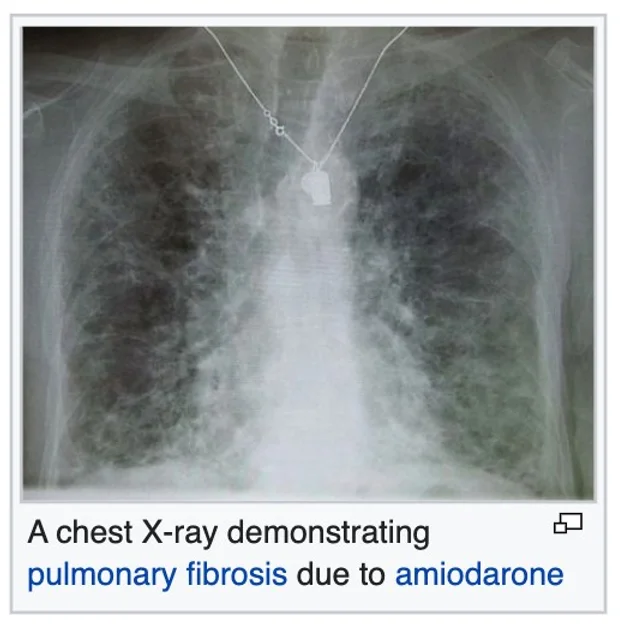
Hamman-Rich syndrome Antisynthetase syndrome
Malignancy
Lymphangitic carcinomatosis
Predominately in children
Diffuse developmental disorders Growth abnormalities deficient alveolarization
Infant conditions of undefined cause
ILD related to alveolar surfactant region
Methodology
Diagnosis of Interstitial Lung Disease and All Other Associated Conditions
Investigation is tailored towards the symptoms and signs. A proper and detailed history looking for the occupational exposures, and for signs of conditions listed above is the first important part of the workup in patients with Interstitial Lung Disease. Pulmonary function tests usually show a restrictive defect with decreased diffusion capacity (DLCO). [9]
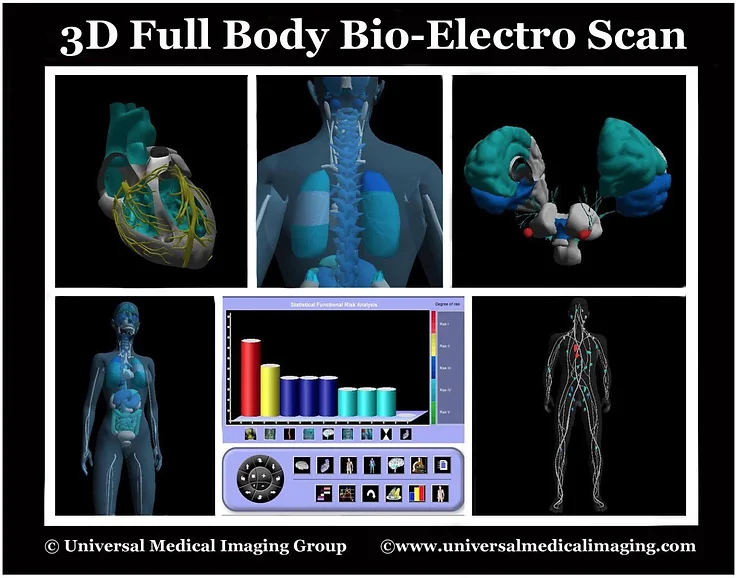
Testing the urine pH, saliva pH, stomach pH, blood pH, and interstitial fluids pH with a complete chemistry are all non-invasive medical tests unique to our 75 years of combined research to discover the cause of ALL sickness and disease, including ones which are idiopathic. The non-invasive medical diagnostics for ITL is called the 3D Full Body Functionality and Interstitial Fluid Testing (FBBES). [10]
New Methods for Whole Body Non-Invasive Diagnostic Testing of the Blood, Interstitial Fluids and Intracellular Fluids
Using 3-D Full Body Bio-Electro Scanning (FBBES), Full Body Thermography (FBT), Full Body Ultrasound (FBU), Live Blood Analysis (LBA) and Dried Blood Analysis (DBA) to Determine the Best Possible Strategy for Preventing and/or Reversing Any Sickness or Disease Condition and to Monitor Effective Treatment Progress [10].
In modern day conventional medicine, surgeons biopsy the lymph nodes and lung tissue to determine how lung disease, including malignancy is spreading to provide staging [11]. Lymphocytes, a type of white blood cell that is found in these lymph nodes which are catch-basins for acidic waste and malignant cells are responsible for breaking-down and removing cellular acidic waste and genetically mutated cells. Impaired lymphocytes and lymph nodes are at least one major factor in the many areas we test for (as described below) physiologically using functionality testing (FBBES) (as described below). The lymphatic system, the lymph nodes and the lymphocytes themselves must be functional in order to prevent and reverse any disease condition, including Interstitial Lung Disease (ILD).
Using electrodes attached to the head, hands, and feet, the functionality of the lymphatic system, circulatory system, muscular system, skeletal system, endocrine system, neurological system, reproductive system, vascular system, digestive system, and respiratory system can be non-invasively analyzed.
Interstitial chemistry, interstitial pH to determine decompensated acidosis, and the electro-conductivity of the cells to determine the state of health of all organs, glands, and tissues in the prevention and reversal of any disease condition, including ILD.
This FBBES also accounts for nutritional deficiencies and metabolic alkalosis or acidosis by measuring the interstitial chemistry, interstitial pH, and the electro-conductivity if the cells of the body. Measuring the pH of the interstitial fluids is more revealing as it pertains to the pH and chemistry than measuring the blood fluids for ILD condition since the blood is always trying to maintain its delicate alkaline pH of 7.365 and will not vary much. Based upon our research we have determined that ILD and all of its many symptoms are expressed as a compromised acidic environment of the interstitial fluids which may negatively affect the state of health of all body cells which make up the organs, glands and tissues, including the lung cells. [12].
It is significantly more important to measure interstitial and intracellular fluids than blood fluids in order to obtain a correct chemistry and pH when making nutritional recommendations in the prevention and treatment of ILD and any malignancy [13].
The following are quantitative measurements in healthy patients, without ILD or malignancy of the lung, comparing blood fluids with intracellular and interstitial fluids of the body compartments as a benchmark to determine deficiencies in alkalizing minerals, protein, and whether or not the patient is in decompensated acidosis of the interstitial fluids, a pre-malignancy, or malignant condition (Note: all ILD and cancer patients are in decompensated interstitial acidosis, low in interstitial sodium and high in interstitial calcium and potassium): [13]
1) Sodium: Na+ mEq/l Venous blood: 130, Arterial blood: 137, Capillary blood: 135, Intracellular fluid: 10 and Interstitial fluid: 135
2) Potassium: K+ mEq/l Venous blood: 3.2, Arterial blood: 3.5, Capillary blood: 4, Intracellular fluid: 140 and Interstitial fluid: 3.17
3) Calcium: Ca++ mEq/l Venous blood: 2.5, Arterial blood: 2.2, Capillary blood: 2.3, Intracellular fluid: 0.0001 and Interstitial fluid: 1.55
4) Magnesium: Mg mEq/l Venous blood: 0.64, Arterial blood: 0.62, Capillary blood: 0.60, Intracellular fluid: 58 and Interstitial fluid: 0.50
5) Chloride: Cl- mEq/l Venous blood: 104, Arterial blood: 101, Capillary blood: 103, Intracellular fluid: 4 and Interstitial fluid: 106
6) Bicarbonate: HCO3 mEq/l Venous blood: 22, Arterial blood: 24, Capillary blood: 23, Intracellular fluid: 10 and Interstitial fluid: 24
7) Phosphorus: P mE/l Venous blood: 2.5, Arterial blood: 2.3, Capillary blood: 2, Intracellular fluid: 75 and Interstitial fluid: 0.70
8) Sulfate: SO4 mEq/l Venous blood: 0.8, Arterial blood: 0.6, Capillary blood: 0.5, Intracellular fluid: 2 and Interstitial fluid: 0
9) Glycemia mg/dl Venous blood: 1, Arterial blood: 1, Capillary blood: 1.01, Intracellular fluid: 0.20 and Interstitial fluid: 0.90
10) Cholesterol mg/dl Venous blood: 0.66, Arterial blood: 0.630, Capillary blood: 0.676, Intracellular fluid: 0.2 and Interstitial fluid: 0.188
11) Partial Pressure of Oxygen or PO2 mmHg Venous blood: 80, Arterial blood: 90, Capillary blood: 89, Intracellular fluid: 20 and Interstitial fluid: 87.2
12) Carbon Dioxide Or PCO2 Venous blood: 46, Arterial blood: 40, Capillary blood: 42, Intracellular fluid: 50 and Interstitial fluid: 46
13) pH or potential of hydrogen Venous blood: 7.36, Arterial blood: 7.4, Capillary blood: 7.38, Intracellular fluid: 7.2 and Interstitial fluid: 7.36
14) Protein g/dl Venous blood: 72, Arterial blood: 74, Capillary blood: 73.7, Intracellular fluid: 68 and interstitial fluid: 20.6
The following are quantitative measurements in healthy patients, without IFLD or malignancy of the lung, comparing blood fluids with intracellular and interstitial fluids of the body compartments as a benchmark to determine deficiencies in alkalizing minerals, protein, and whether or not the patient is in decompensated acidosis of the interstitial fluids, a pre-malignancy, or malignant condition (Note: all ILD and cancer patients are in decompensated interstitial acidosis, low in interstitial sodium and high in interstitial calcium and potassium): [13]
Results
Using non-invasive 3D-FBBES for determining the chemistry of the interstitial fluids of the whole body, including the lungs is not only a test for determining decompensated acidosis or compensated alkalosis (a pre-condition of IFLD) of the ALL interstitial fluids of the body, including the lungs, but is an effective way to determine efficacy of any medical or natural alkalizing therapy in prevention and treatment of ANY sickness or disease. The results of the using 3D-GBBES combined with conventional medical blood tests and/or the Non-Invasive Blood Tests [NIBT] covering over 170 parameters of the blood, including blood counts, chemistry, pH of the blood, stomach and most important quantifying acid loads, such as lactic acid with a 98 percent accuracy for determining pre-interstitial-fluid lung disease and IFLD and its many symptomologies as listed above.
References
1. GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). “Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015”. Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282. 2. GBD 2015 Mortality and Causes of Death, Collaborators. (8 October 2016). “Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015”. Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281. 3. King TE (August 2005). “Clinical advances in the diagnosis and therapy of the interstitial lung diseases”. American Journal of Respiratory and Critical Care Medicine. 172 (3): 268–79. doi:10.1164/rccm.200503-483OE. PMID 15879420. 4. “Frequently Asked Questions About Interstitial Lung Disease”. University of Chicago Medical Center.
5. Young RO, Migalko G (2015) Alkalizing Nutritional Therapy in the Prevention and Reversal of any Cancerous Condition. Int J Complement Alt Med 2(1): 00046. DOI: 10.15406/ijcam.2015.02.00046 6. Bush A, Cunningham S, de Blic J, Barbato A, Clement A, Epaud R, Hengst M, Kiper N, Nicholson AG, Wetzke M, Snijders D, Schwerk N, Griese M (November 2015). “European protocols for the diagnosis and initial treatment of interstitial lung disease in children”. review. Thorax. 70 (11): 1078–84. doi:10.1136/thoraxjnl-2015-207349. PMID 26135832.
7. Young Robert, Young Shelley (2010) The pH Miracle. Balance Your Diet, Reclaim Your Health. Hachett Publishing, Haryana, India. 8. Bourke SJ (August 2006). “Interstitial lung disease: progress and problems”. Postgraduate Medical Journal. 82 (970): 494–9. doi:10.1136/pgmj.2006.046417. PMC 2585700. PMID 16891438. 9. Ryu JH, Olson EJ, Midthun DE, Swensen SJ (November 2002). “Diagnostic approach to the patient with diffuse lung disease”. Mayo Clinic Proceedings. 77 (11): 1221–7, quiz 1227. doi:10.4065/77.11.1221. PMID 12440558. 10. Galina Migalko, Universal Medical Imaging Group, Valley Village, California, USA. www.universalmedicalimaging.com
11. Ronald G.CrystalM.D., James E.GadekM.D., Victor J.FerransM.D., Jack D.FulmerM.D., Bruce R.LineM.D., Gary W.HunninghakeM.D, “Review Interstitial lung disease: Current concepts of pathogenesis, staging and therapy”. The American Journal of Medicine
Volume 70, Issue 3, March 1981, Pages 542-568.
12. Rose BD (2001) Post TW. Clinical Physiology of Acid-Base and Electrolyte Disorders, (5th edn), McGraw-Hill, New York. p. 347.
13. Young RO, Migalko G (2015) Alkalizing Nutritional Therapy in the Prevention and Reversal of any Cancerous Condition. Int J Complement Alt Med 2(1): 00046. DOI: 10.15406/ijcam.2015.02.00046.



