Genetic BioEngineering – Writing the Circuitry of HUMAN CELLS for 5G AI Super Computer Connection!
Updated: Nov 7, 2022
Biden Ushers in Transhumanism for ALL Americans With His Executive Order
September 22, 2022

Executive Order on Advancing Biotechnology and Bio
Manufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy
-
HOME
-
BRIEFING ROOM
-
PRESIDENTIAL ACTIONS
By the authority vested in me as President by the Constitution and the laws of the United States of America, it is hereby ordered as follows:
Section 1. Policy
It is the policy of my Administration to coordinate a whole-of-government approach to advance biotechnology and biomanufacturing towards innovative solutions in health, climate change, energy, food security, agriculture, supply chain resilience, and national and economic security.
Central to this policy and its outcomes are principles of equity, ethics, safety, and security that enable access to technologies, processes, and products in a manner that benefits all Americans and the global community and that maintains United States technological leadership and economic competitiveness.
Biotechnology harnesses the power of biology to create new services and products, which provide opportunities to grow the United States economy and workforce and improve the quality of our lives and the environment. The economic activity derived from biotechnology and biomanufacturing is referred to as “the bioeconomy.” The COVID-19 pandemic has demonstrated the vital role of biotechnology and biomanufacturing in developing and producing life-saving diagnostics, therapeutics, and vaccines that protect Americans and the world. Although the power of these technologies is most vivid at the moment in the context of human health, biotechnology and biomanufacturing can also be used to achieve our climate and energy goals, improve food security and sustainability, secure our supply chains, and grow the economy across all of America.
For biotechnology and biomanufacturing to help us achieve our societal goals, the United States needs to invest in foundational scientific capabilities. We need to develop genetic engineering technologies and techniques to be able to write circuitry for cells and predictably program biology in the same way in which we write software and program computers; unlock the power of biological data, including through computing tools and artificial intelligence; and advance the science of scale‑up production while reducing the obstacles for commercialization so that innovative technologies and products can reach markets faster.
Simultaneously, we must take concrete steps to reduce biological risks associated with advances in biotechnology. We need to invest in and promote biosafety and biosecurity to ensure that biotechnology is developed and deployed in ways that align with United States principles and values and international best practices, and not in ways that lead to accidental or deliberate harm to people, animals, or the environment. In addition, we must safeguard the United States bioeconomy, as foreign adversaries and strategic competitors alike use legal and illegal means to acquire United States technologies and data, including biological data, and proprietary or precompetitive information, which threatens United States economic competitiveness and national security.
We also must ensure that uses of biotechnology and biomanufacturing are ethical and responsible; are centered on a foundation of equity and public good, consistent with Executive Order 13985 of January 20, 2021 (Advancing Racial Equity and Support for Underserved Communities Through the Federal Government); and are consistent with respect for human rights. Resources should be invested justly and equitably so that biotechnology and biomanufacturing technologies benefit all Americans, especially those in underserved communities, as well as the broader global community.
To achieve these objectives, it is the policy of my Administration to:
(a) bolster and coordinate Federal investment in key research and development (R&D) areas of biotechnology and biomanufacturing in order to further societal goals;
(b) foster a biological data ecosystem that advances biotechnology and biomanufacturing innovation, while adhering to principles of security, privacy, and responsible conduct of research;
(c) improve and expand domestic biomanufacturing production capacity and processes, while also increasing piloting and prototyping efforts in biotechnology and biomanufacturing to accelerate the translation of basic research results into practice;
(d) boost sustainable biomass production and create climate-smart incentives for American agricultural producers and forest landowners;
(e) expand market opportunities for bioenergy and biobased products and services;
(f) train and support a diverse, skilled workforce and a next generation of leaders from diverse groups to advance biotechnology and biomanufacturing;
(g) clarify and streamline regulations in service of a science- and risk-based, predictable, efficient, and transparent system to support the safe use of products of biotechnology;
(h) elevate biological risk management as a cornerstone of the life cycle of biotechnology and biomanufacturing R&D, including by providing for research and investment in applied biosafety and biosecurity innovation;
(i) promote standards, establish metrics, and develop systems to grow and assess the state of the bioeconomy; to better inform policy, decision-making, and investments in the bioeconomy; and to ensure equitable and ethical development of the bioeconomy;
(j) secure and protect the United States bioeconomy by adopting a forward‑looking, proactive approach to assessing and anticipating threats, risks, and potential vulnerabilities (including digital intrusion, manipulation, and exfiltration efforts by foreign adversaries), and by partnering with the private sector and other relevant stakeholders to jointly mitigate risks to protect technology leadership and economic competitiveness; and
(k) engage the international community to enhance biotechnology R&D cooperation in a way that is consistent with United States principles and values and that promotes best practices for safe and secure biotechnology and biomanufacturing research, innovation, and product development and use. The efforts undertaken pursuant to this order to further these policies shall be referred to collectively as the National Biotechnology and Biomanufacturing Initiative.
Sec. 2. Coordination
The Assistant to the President for National Security Affairs (APNSA), in consultation with the Assistant to the President for Economic Policy (APEP) and the Director of the Office of Science and Technology Policy (OSTP), shall coordinate the executive branch actions necessary to implement this order through the interagency process described in National Security Memorandum 2 of February 4, 2021 (Renewing the National Security Council System) (NSM-2 process). In implementing this order, heads of agencies (as defined in section 13 of this order) shall, as appropriate and consistent with applicable law, consult outside stakeholders, such as those in industry; academia; nongovernmental organizations; communities; labor unions; and State, local, Tribal, and territorial governments to advance the policies described in section 1 of this order.
Sec. 3. Harnessing Biotechnology and Biomanufacturing R&D to Further Societal Goals
(a) Within 180 days of the date of this order, the heads of agencies specified in subsections (a)(i)-(v) of this section shall submit the following reports on biotechnology and biomanufacturing to further societal goals related to health, climate change and energy, food and agricultural innovation, resilient supply chains, and cross-cutting scientific advances. The reports shall be submitted to the President through the APNSA, in coordination with the Director of the Office of Management and Budget (OMB), the APEP, the Assistant to the President for Domestic Policy (APDP), and the Director of OSTP.
(i) The Secretary of Health and Human Services (HHS), in consultation with the heads of appropriate agencies as determined by the Secretary, shall submit a report assessing how to use biotechnology and biomanufacturing to achieve medical breakthroughs, reduce the overall burden of disease, and improve health outcomes.
(ii) The Secretary of Energy, in consultation with the heads of appropriate agencies as determined by the Secretary, shall submit a report assessing how to use biotechnology, biomanufacturing, bioenergy, and biobased products to address the causes and adapt to and mitigate the impacts of climate change, including by sequestering carbon and reducing greenhouse gas emissions.
(iii) The Secretary of Agriculture, in consultation with the heads of appropriate agencies as determined by the Secretary, shall submit a report assessing how to use biotechnology and biomanufacturing for food and agriculture innovation, including by improving sustainability and land conservation; increasing food quality and nutrition; increasing and protecting agricultural yields; protecting against plant and animal pests and diseases; and cultivating alternative food sources.
(iv) The Secretary of Commerce, in consultation with the Secretary of Defense, the Secretary of HHS, and the heads of other appropriate agencies as determined by the Secretary of Commerce, shall submit a report assessing how to use biotechnology and biomanufacturing to strengthen the resilience of United States supply chains.
(v) The Director of the National Science Foundation (NSF), in consultation with the heads of appropriate agencies as determined by the Director, shall submit a report identifying high-priority fundamental and use‑inspired basic research goals to advance biotechnology and biomanufacturing and to address the societal goals identified in this section.
(b) Each report specified in subsection (a) of this section shall identify high-priority basic research and technology development needs to achieve the overall objectives described in subsection (a) of this section, as well as opportunities for public-private collaboration. Each of these reports shall also include recommendations for actions to enhance biosafety and biosecurity to reduce risk throughout the biotechnology R&D and biomanufacturing lifecycles.
(c) Within 100 days of receiving the reports required under subsection (a) of this section, the Director of OSTP, in coordination with the Director of OMB, the APNSA, the APEP, the APDP, and the heads of appropriate agencies as determined through the NSM-2 process, shall develop a plan (implementation plan) to implement the recommendations in the reports. The development of this implementation plan shall also include the solicitation of input from external experts regarding potential ethical implications or other societal impacts, including environmental sustainability and environmental justice, of the recommendations contained in the reports required under subsection (a) of this section. The implementation plan shall include assessments and make recommendations regarding any such implications or impacts.
(d) Within 90 days of the date of this order, the Director of OMB, in consultation with the heads of appropriate agencies as determined through the NSM-2 process, shall perform a budget crosscut to identify existing levels of agency spending on biotechnology- and biomanufacturing-related activities to inform the development of the implementation plan described in subsection (c) of this section.
(e) The APNSA, in coordination with the Director of OMB, the APEP, the APDP, and the Director of OSTP, shall review the reports required under subsection (a) of this section and shall submit the reports to the President in an unclassified form, but may include a classified annex.
(f) The APNSA, in coordination with the Director of OMB, the APEP, the APDP, and the Director of OSTP, shall include a cover memorandum for the reports submitted pursuant to subsection (a) of this section, along with the implementation plan required under subsection (c) of this section, in which they make any additional overall recommendations for advancing biotechnology and biomanufacturing.
(g) Within 2 years of the date of this order, agencies at which recommendations are directed in the implementation plan required under subsection (c) of this section shall report to the Director of OMB, the APNSA, the APEP, the APDP, and the Director of OSTP on measures taken and resources allocated to enhance biotechnology and biomanufacturing, consistent with the implementation plan described in subsection (c) of this section.
(h) Within 180 days of the date of this order, the President’s Council of Advisors on Science and Technology shall submit to the President and make publicly available a report on the bioeconomy that provides recommendations on how to maintain United States competitiveness in the global bioeconomy.
Sec. 4. Data for the Bioeconomy
(a) In order to facilitate development of the United States bioeconomy, my Administration shall establish a Data for the Bioeconomy Initiative (Data Initiative) that will ensure that high-quality, wide-ranging, easily accessible, and secure biological data sets can drive breakthroughs for the United States bioeconomy.
To assist in the development of the Data Initiative, the Director of OSTP, in coordination with the Director of OMB and the heads of appropriate agencies as determined by the Director of OSTP, and in consultation with external stakeholders, shall issue a report within 240 days of the date of this order that:
(i) identifies the data types and sources, to include genomic and multiomic information, that are most critical to drive advances in health, climate, energy, food, agriculture, and biomanufacturing, as well as other bioeconomy-related R&D, along with any data gaps;
(ii) sets forth a plan to fill any data gaps and make new and existing public data findable, accessible, interoperable, and reusable in ways that are equitable, standardized, secure, and transparent, and that are integrated with platforms that enable the use of advanced computing tools;
(iii) identifies — based on the data types and sources described in subsection (a)(i) of this section — security, privacy, and other risks (such as malicious misuses, manipulation, exfiltration, and deletion), and provides a data-protection plan to mitigate these risks; and
(iv) outlines the Federal resources, legal authorities, and actions needed to support the Data Initiative and achieve the goals outlined in this subsection, with a timeline for action.
(b) The Secretary of Homeland Security, in coordination with the Secretary of Defense, the Secretary of Agriculture, the Secretary of Commerce (acting through the Director of the National Institute of Standards and Technology (NIST)), the Secretary of HHS, the Secretary of Energy, and the Director of OMB, shall identify and recommend relevant cybersecurity best practices for biological data stored on Federal Government information systems, consistent with applicable law and Executive Order 14028 of May 12, 2021 (Improving the Nation’s Cybersecurity).
(c) The Secretary of Commerce, acting through the Director of NIST and in coordination with the Secretary of HHS, shall consider bio-related software, including software for laboratory equipment, instrumentation, and data management, in establishing baseline security standards for the development of software sold to the United States Government, consistent with section 4 of Executive Order 14028.
Sec. 5. Building a Vibrant Domestic Biomanufacturing Ecosystem
(a) Within 180 days of the date of this order, the APNSA and the APEP, in coordination with the Secretary of Defense, the Secretary of Agriculture, the Secretary of Commerce, the Secretary of HHS, the Secretary of Energy, the Director of NSF, and the Administrator of the National Aeronautics and Space Administration (NASA), shall develop a strategy that identifies policy recommendations to expand domestic biomanufacturing capacity for products spanning the health, energy, agriculture, and industrial sectors, with a focus on advancing equity, improving biomanufacturing processes, and connecting relevant infrastructure. Additionally, this strategy shall identify actions to mitigate risks posed by foreign adversary involvement in the biomanufacturing supply chain and to enhance biosafety, biosecurity, and cybersecurity in new and existing infrastructure.
(b) Agencies identified in subsections (b)(i)-(iv) of this section shall direct resources, as appropriate and consistent with applicable law, towards the creation or expansion of programs that support a vibrant domestic biomanufacturing ecosystem, as informed by the strategy developed pursuant to subsection (a) of this section:
(i) the NSF shall expand its existing Regional Innovation Engine program to advance emerging technologies, including biotechnology;
(ii) the Department of Commerce shall address challenges in biomanufacturing supply chains and related biotechnology development infrastructure;
(iii) the Department of Defense shall incentivize the expansion of domestic, flexible industrial biomanufacturing capacity for a wide range of materials that can be used to make a diversity of products for the defense supply chain; and
(iv) the Department of Energy shall support research to accelerate bioenergy and bioproduct science advances, to accelerate biotechnology and bioinformatics tool development, and to reduce the hurdles to commercialization, including through incentivizing the engineering scale-up of promising biotechnologies and the expansion of biomanufacturing capacity.
(c) Within 1 year of the date of this order, the Secretary of Agriculture, in consultation with the heads of appropriate agencies as determined by the Secretary, shall submit a plan to the President, through the APNSA and the APEP, to support the resilience of the United States biomass supply chain for domestic biomanufacturing and biobased product manufacturing, while also advancing food security, environmental sustainability, and the needs of underserved communities. This plan shall include programs to encourage climate-smart production and use of domestic biomass, along with budget estimates, including accounting for funds appropriated for Fiscal Year (FY) 2022 and proposed in the President’s FY 2023 Budget.
(d) Within 180 days of the date of this order, the Secretary of Homeland Security, in coordination with the heads of appropriate agencies as determined by the Secretary, shall:
(i) provide the APNSA with vulnerability assessments of the critical infrastructure and national critical functions associated with the bioeconomy, including cyber, physical, and systemic risks, and recommendations to secure and make resilient these components of our infrastructure and economy; and
(ii) enhance coordination with industry on threat information sharing, vulnerability disclosure, and risk mitigation for cybersecurity and infrastructure risks to the United States bioeconomy, including risks to biological data and related physical and digital infrastructure and devices. This coordination shall be informed in part by the assessments described in subsection (d)(i) of this section.
Sec. 6. Biobased Products Procurement.
(a) Consistent with the requirements of 7 U.S.C. 8102, within 1 year of the date of this order, procuring agencies as defined in 7 U.S.C. 8102(a)(1)(A) that have not yet established a biobased procurement program as described in 7 U.S.C. 8102(a)(2) shall establish such a program.
(b) Procuring agencies shall require that, within 2 years of the date of this order, all appropriate staff (including contracting officers, purchase card managers, and purchase card holders) complete training on biobased product purchasing. The Office of Federal Procurement Policy, within OMB, in cooperation with the Secretary of Agriculture, shall provide training materials for procuring agencies.
(c) Within 180 days of the date of this order and annually thereafter, procuring agencies shall report previous fiscal year spending to the Director of OMB on the following:
(i) the number and dollar value of contracts entered into during the previous fiscal year that include the direct procurement of biobased products;
(ii) the number of service and construction (including renovations) contracts entered into during the previous fiscal year that include language on the use of biobased products; and
(iii) the types and dollar values of biobased products actually used by contractors in carrying out service and construction (including renovations) contracts during the previous fiscal year.
(d) The requirements in subsection (c) of this section shall not apply to purchase card transactions and other “[a]ctions not reported” to the Federal Procurement Data System pursuant to 48 CFR 4.606(c).
(e) Within 1 year of the date of this order and annually thereafter, the Director of OMB shall publish information on biobased procurement resulting from the data collected under subsection (c) of this section and information reported under 7 U.S.C. 8102, along with other related information, and shall use scorecards or similar systems to encourage increased biobased purchasing.
(f) Within 1 year of the date of this order and annually thereafter, procuring agencies shall report to the Secretary of Agriculture specific categories of biobased products that are unavailable to meet their procurement needs, along with desired performance standards for currently unavailable products and other relevant specifications. The Secretary of Agriculture shall publish this information annually. When new categories of biobased products become commercially available, the Secretary of Agriculture shall designate new product categories for preferred Federal procurement, as prescribed by 7 U.S.C. 8102.
(g) Procuring agencies shall strive to increase by 2025 the amount of biobased product obligations or the number or dollar value of biobased-only contracts, as reflected in the information described in subsection (c) of this section, and as appropriate and consistent with applicable law.
Sec. 7. Biotechnology and Biomanufacturing Workforce.
(a) The United States Government shall expand training and education opportunities for all Americans in biotechnology and biomanufacturing. To support this objective, within 200 days of the date of this order, the Secretary of Commerce, the Secretary of Labor, the Secretary of Education, the APDP, the Director of OSTP, and the Director of NSF shall produce and make publicly available a plan to coordinate and use relevant Federal education and training programs, while also recommending new efforts to promote multi-disciplinary education programs. This plan shall promote the implementation of formal and informal education and training (such as opportunities at technical schools and certificate programs), career and technical education, and expanded career pathways into existing degree programs for biotechnology and biomanufacturing. This plan shall also include a focused discussion of Historically Black Colleges and Universities, Tribal Colleges and Universities, and Minority Serving Institutions and the extent to which agencies can use existing statutory authorities to promote racial and gender equity and support underserved communities, consistent with the policy established in Executive Order 13985.
Finally, this plan shall account for funds appropriated for FY 2022 and proposed in the President’s FY 2023 Budget.
(b) Within 2 years of the date of this order, agencies that support relevant Federal education and training programs as described in subsection (a) of this section shall report to the President through the APNSA, in coordination with the Director of OMB, the ADPD, and the Director of OSTP, on measures taken and resources allocated to enhance workforce development pursuant to the plan described in subsection (a) of this section.
Sec. 8. Biotechnology Regulation Clarity and Efficiency
Advances in biotechnology are rapidly altering the product landscape. The complexity of the current regulatory system for biotechnology products can be confusing and create challenges for businesses to navigate.
To improve the clarity and efficiency of the regulatory process for biotechnology products, and to enable products that further the societal goals identified in section 3 of this order, the Secretary of Agriculture, the Administrator of the Environmental Protection Agency, and the Commissioner of Food and Drugs, in coordination with the Director of OMB, the ADPD, and the Director of OSTP, shall:
(a) within 180 days of the date of this order, identify areas of ambiguity, gaps, or uncertainties in the January 2017 Update to the Coordinated Framework for the Regulation of Biotechnology or in the policy changes made pursuant to Executive Order 13874 of June 11, 2019 (Modernizing the Regulatory Framework for Agricultural Biotechnology Products), including by engaging with developers and external stakeholders, and through horizon scanning for novel products of biotechnology;
(b) within 100 days of completing the task in subsection (a) of this section, provide to the general public plain-language information regarding the regulatory roles, responsibilities, and processes of each agency, including which agency or agencies are responsible for oversight of different types of products developed with biotechnology, with case studies, as appropriate;
(c) within 280 days of the date of this order, provide a plan to the Director of OMB, the ADPD, and the Director of OSTP with processes and timelines to implement regulatory reform, including identification of the regulations and guidance documents that can be updated, streamlined, or clarified; and identification of potential new guidance or regulations, where needed;
(d) within 1 year of the date of this order, build on the Unified Website for Biotechnology Regulation developed pursuant to Executive Order 13874 by including on the website the information developed under subsection (b) of this section, and by enabling developers of biotechnology products to submit inquiries about a particular product and promptly receive a single, coordinated response that provides, to the extent practicable, information and, when appropriate, informal guidance regarding the process that the developers must follow for Federal regulatory review; and
(e) within 1 year of the date of this order, and annually thereafter for a period of 3 years, provide an update regarding progress in implementing this section to the Director of OMB, the United States Trade Representative (USTR), the APNSA, the ADPD, and the Director of OSTP. Each 1-year update shall identify any gaps in statutory authority that should be addressed to improve the clarity and efficiency of the regulatory process for biotechnology products, and shall recommend additional executive actions and legislative proposals to achieve such goals.
Sec. 9. Reducing Risk by Advancing Biosafety and Biosecurity
(a) The United States Government shall launch a Biosafety and Biosecurity Innovation Initiative, which shall seek to reduce biological risks associated with advances in biotechnology, biomanufacturing, and the bioeconomy. Through the Biosafety and Biosecurity Innovation Initiative — which shall be established by the Secretary of HHS, in coordination with the heads of other relevant agencies as determined by the Secretary — agencies that fund, conduct, or sponsor life sciences research shall implement the following actions, as appropriate and consistent with applicable law:
(i) support, as a priority, investments in applied biosafety research and innovations in biosecurity to reduce biological risk throughout the biotechnology R&D and biomanufacturing lifecycles; and
(ii) use Federal investments in biotechnology and biomanufacturing to incentivize and enhance biosafety and biosecurity practices and best practices throughout the United States and international research enterprises.
(b) Within 180 days of the date of this order, the Secretary of HHS and the Secretary of Homeland Security, in coordination with agencies that fund, conduct, or sponsor life sciences research, shall produce a plan for biosafety and biosecurity for the bioeconomy, including recommendations to:
(i) enhance applied biosafety research and bolster innovations in biosecurity to reduce risk throughout the biotechnology R&D and biomanufacturing lifecycles; and
(ii) use Federal investments in biological sciences, biotechnology, and biomanufacturing to enhance biosafety and biosecurity best practices throughout the bioeconomy R&D enterprise.
(c) Within 1 year of the date of this order, agencies that fund, conduct, or sponsor life sciences research shall report to the APNSA, through the Assistant to the President and Homeland Security Advisor, on efforts to achieve the objectives described in subsection (a) of this section.
Sec. 10. Measuring the Bioeconomy
(a) Within 90 days of the date of this order, the Secretary of Commerce, through the Director of NIST, shall, in consultation with other agencies as determined by the Director, industry, and other stakeholders, as appropriate, create and make publicly available a lexicon for the bioeconomy, with consideration of relevant domestic and international definitions and with the goal of assisting in the development of measurements and measurement methods for the bioeconomy that support uses such as economic measurement, risk assessments, and the application of machine learning and other artificial intelligence tools.
(b) The Chief Statistician of the United States, in coordination with the Secretary of Agriculture, the Secretary of Commerce, the Director of NSF, and the heads of other appropriate agencies as determined by the Chief Statistician, shall improve and enhance Federal statistical data collection designed to characterize the economic value of the United States bioeconomy, with a focus on the contribution of biotechnology to the bioeconomy. This effort shall include:
(i) within 180 days of the date of this order, assessing, through the Department of Commerce’s Bureau of Economic Analysis, the feasibility, scope, and costs of developing a national measurement of the economic contributions of the bioeconomy, and, in particular, the contributions of biotechnology to the bioeconomy, including recommendations and a plan for next steps regarding whether development of such a measurement should be pursued; and (ii) within 120 days of the date of this order, establishing an Interagency Technical Working Group (ITWG), chaired by the Chief Statistician of the United States, which shall include representatives of the Department of Agriculture, the Department of Commerce, OSTP, the NSF, and other appropriate agencies as determined by the Chief Statistician of the United States.
(A) Within 1 year of the date of this order, the ITWG shall recommend bioeconomy-related revisions to the North American Industry Classification System (NAICS) and the North American Product Classification System (NAPCS) to the Economic Classification Policy Committee. In 2026, the ITWG shall initiate a review process of the 2023 recommendations and update the recommendations, as appropriate, to provide input to the 2027 NAICS and NAPCS revision processes.
(B) Within 18 months of the date of this order, the ITWG shall provide a report to the Chief Statistician of the United States describing the Federal statistical collections of information that take advantage of bioeconomy-related NAICS and NAPCS codes, and shall include recommendations to implement any bioeconomy-related changes as part of the 2022 revisions of the NAICS and NAPCS. As part of its work, the ITWG shall consult with external stakeholders.
Sec. 11. Assessing Threats to the United States Bioeconomy
(a) The Director of National Intelligence (DNI) shall lead a comprehensive interagency assessment of ongoing, emerging, and future threats to United States national security from foreign adversaries against the bioeconomy and from foreign adversary development and application of biotechnology and biomanufacturing, including acquisition of United States capabilities, technologies, and biological data.
As part of this effort, the DNI shall work closely with the Department of Defense to assess technical applications of biotechnology and biomanufacturing that could be misused by a foreign adversary for military purposes or that could otherwise pose a risk to the United States. In support of these objectives, the DNI shall identify elements of the bioeconomy of highest concern and establish processes to support ongoing threat identification and impact assessments.
(b) Within 240 days of the date of this order, the DNI shall provide classified assessments to the APNSA related to:
(i) threats to United States national and economic security posed by foreign adversary development and application of biomanufacturing; and
(ii) foreign adversary means of, and intended usages related to, acquisition of United States biotechnologies, biological data, and proprietary or precompetitive information.
(c) Within 120 days of receiving the DNI’s assessments, the APNSA shall coordinate with the heads of relevant agencies as determined through the NSM-2 process to develop and finalize a plan to mitigate risks to the United States bioeconomy, based upon the threat identification and impact assessments described in subsection (a) of this section, the vulnerability assessments described in section 5(d) of this order, and other relevant assessments or information. The plan shall identify where executive action, regulatory action, technology protection, or statutory authorities are needed to mitigate these risks in order to support the technology leadership and economic competitiveness of the United States bioeconomy.
(d) The United States Government contracts with a variety of providers to support its functioning, including by contracting for services related to the bioeconomy. It is important that these contracts are awarded according to full and open competition, as consistent with the Competition in Contracting Act of 1984 (Public Law 98-369, 98 Stat. 1175). In accordance with these objectives, and within 1 year of the date of this order, the Director of OSTP, in coordination with the Secretary of Defense, the Attorney General, the Secretary of HHS, the Secretary of Energy, the Secretary of Homeland Security, the DNI, the Administrator of NASA, and the Administrator of General Services, shall review the national security implications of existing requirements related to Federal procurement — including requirements contained in the Federal Acquisition Regulation (FAR) and the Defense Federal Acquisition Regulation Supplement — and shall recommend updates to those requirements to the FAR Council, the Director of OMB, and the heads of other appropriate agencies as determined through the NSM-2 process. The recommendations shall aim to standardize pre-award data collection to enable due diligence review of conflict of interest; conflict of commitment; foreign ownership, control, or influence; or other potential national security concerns.
The recommendations shall also include legislative proposals, as relevant.
(e) The Director of OMB shall issue a management memorandum to agencies, or take other appropriate action, to provide generalized guidance based on the recommendations received pursuant to subsection (d) of this section.
Sec. 12. International Engagement
(a) The Department of State and other agencies that engage with international partners as part of their missions shall undertake the following actions with foreign partners, as appropriate and consistent with applicable law — with a specific focus on developing countries, international organizations, and nongovernmental entities — to promote and protect both the United States and global bioeconomies:
(i) enhance cooperation, including joint research projects and expert exchanges, on biotechnology R&D, especially in genomics;
(ii) encourage regulatory cooperation and the adoption of best practices to evaluate and promote innovative products, with an emphasis on those practices and products that support sustainability and climate objectives;
(iii) develop joint training arrangements and initiatives to support bioeconomy jobs in the United States;
(iv) work to promote the open sharing of scientific data, including genetic sequence data, to the greatest extent possible in accordance with applicable law and policy, while seeking to ensure that any applicable access and benefit-sharing mechanisms do not hinder the rapid and sustainable development of innovative products and biotechnologies;
(v) conduct horizon scanning to anticipate threats to the global bioeconomy, including national security threats from foreign adversaries acquiring sensitive technologies or data, or disrupting essential bio-related supply chains, and to identify opportunities to address those threats;
(vi) engage allies and partners to address shared national security threats;
(vii) develop, and work to promote and implement, biosafety and biosecurity best practices, tools, and resources bilaterally and multilaterally to facilitate appropriate oversight for life sciences, dual-use research of concern, and research involving potentially pandemic and other high-consequence pathogens, and to enhance sound risk management of biotechnology- and biomanufacturing-related R&D globally; and
(viii) explore how to align international classifications of biomanufactured products, as appropriate, to measure the value of those products to both the United States and global bioeconomies.
(b) Within 180 days of the date of this order, the Secretary of State, in coordination with the USTR and the heads of other agencies as determined by the Secretary, as appropriate, shall submit to the APNSA a plan to support the objectives described in subsection (a) of this section with foreign partners, international organizations, and nongovernmental entities.
Sec. 13. Definitions. For purposes of this order:
(a) The term “agency” has the meaning given that term by 44 U.S.C. 3502(1).
(b) The term “biotechnology” means technology that applies to or is enabled by life sciences innovation or product development.
(c) The term “biomanufacturing” means the use of biological systems to develop products, tools, and processes at commercial scale.
(d) The term “bioeconomy” means economic activity derived from the life sciences, particularly in the areas of biotechnology and biomanufacturing, and includes industries, products, services, and the workforce.
(e) The term “biological data” means the information, including associated descriptors, derived from the structure, function, or process of a biological system(s) that is measured, collected, or aggregated for analysis.
(f) The term “biomass” means any material of biological origin that is available on a renewable or recurring basis. Examples of biomass include plants, trees, algae, and waste material such as crop residue, wood waste, animal waste and byproducts, food waste, and yard waste.
(g) The term “biobased product” has the meaning given that term in 7 U.S.C. 8101(4).
(h) The term “bioenergy” means energy derived in whole or in significant part from biomass.
(i) The term “multiomic information” refers to combined information derived from data, analysis, and interpretation of multiple omics measurement technologies to identify or analyze the roles, relationships, and functions of biomolecules (including nucleic acids, proteins, and metabolites) that make up a cell or cellular system. Omics are disciplines in biology that include genomics, transcriptomics, proteomics, and metabolomics.
(j) The term “key R&D areas” includes fundamental R&D of emerging biotechnologies, including engineering biology; predictive engineering of complex biological systems, including the designing, building, testing, and modeling of entire living cells, cell components, or cellular systems; quantitative and theory-driven multi-disciplinary research to maximize convergence with other enabling technologies; and regulatory science, including the development of new information, criteria, tools, models, and approaches to inform and assist regulatory decision-making. These R&D priorities should be coupled with advances in predictive modeling, data analytics, artificial intelligence, bioinformatics, high-performance and other advanced computing systems, metrology and data-driven standards, and other non-life science enabling technologies.
(k) The terms “equity” and “underserved communities” have the meanings given those terms by sections 2(a) and 2(b) of Executive Order 13985. (l) The term “Tribal Colleges and Universities” has the meaning given that term by section 5(e) of Executive Order 14049 of October 11, 2021 (White House Initiative on Advancing Educational Equity, Excellence, and Economic Opportunity for Native Americans and Strengthening Tribal Colleges and Universities).
(m) The term “Historically Black Colleges and Universities” has the meaning given that term by section 4(b) of Executive Order 14041 of September 3, 2021 (White House Initiative on Advancing Educational Equity, Excellence, and Economic Opportunity Through Historically Black Colleges and Universities).
(n) The term “minority serving institution” has the meaning given that term by 38 U.S.C. 3698(f)(4).
(o) The term “foreign adversary” has the meaning given that term by section 3(b) of Executive Order 14034 of June 9, 2021 (Protecting Americans’ Sensitive Data From Foreign Adversaries).
(p) The term “life sciences” means all sciences that study or use living organisms, viruses, or their products, including all disciplines of biology and all applications of the biological sciences (including biotechnology, genomics, proteomics, bioinformatics, and pharmaceutical and biomedical research and techniques), but excluding scientific studies associated with radioactive materials or toxic chemicals that are not of biological origin or synthetic analogues of toxins.
Sec. 14. General Provisions
(a) Nothing in this order shall be construed to impair or otherwise affect:
(i) the authority granted by law to an executive department or agency, or the head thereof; or
(ii) the functions of the Director of OMB relating to budgetary, administrative, or legislative proposals.
(b) This order shall be implemented consistent with applicable law and subject to the availability of appropriations.
(c) This order is not intended to, and does not, create any right or benefit, substantive or procedural, enforceable at law or in equity by any party against the United States, its departments, agencies, or entities, its officers, employees, or agents, or any other person.
JOSEPH R. BIDEN JR. THE WHITE HOUSE, September 12, 2022.

Related


Biden’s Transhumanism Agenda Hidden in Plain Site With His Executive Order Signed September 22, 2022!
Transfection to Transhumanism – Part 1 – https://rumble.com/v1n1ack-transfection-to-transhumanism-part-1.html

Transfection to Transhumanism – Part 2 – https://rumble.com/v1n1dgi-transfection-to-transhumanism-part-2.html
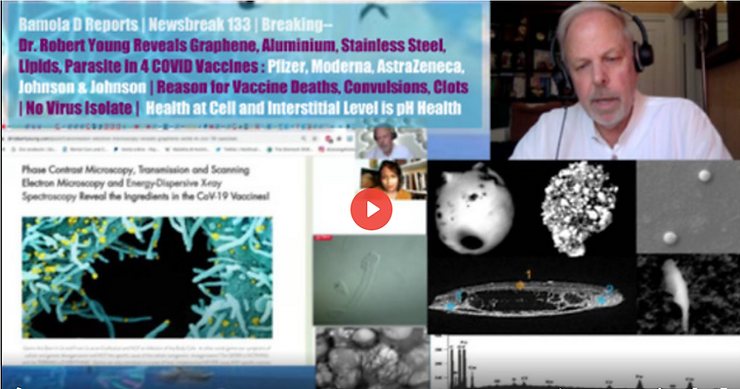
You should know that reduced graphene oxide is different from almost every other material now known, and this difference is why eugenicists, technocrats and corrupt governments want it in everything; water, air, drugs, vaxxines, etc.
Protect Yourself From Being Lockstep From Graphene Activated by 4 & 5G!

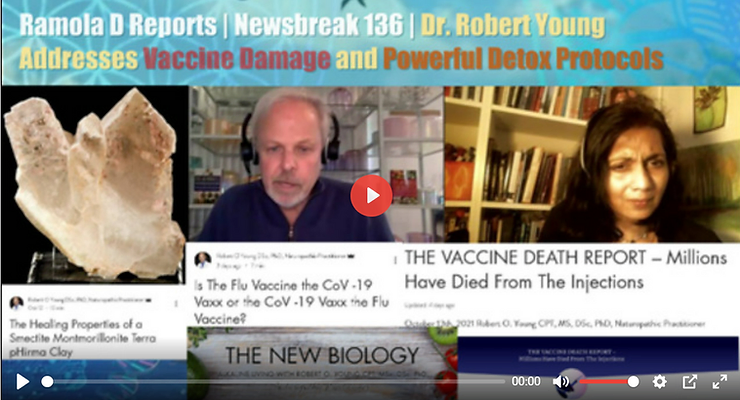
To learn more about Dr. Robert O. Young go to: www.drrobertyoung.com/blog

Dr. Robert O. Young has been widely recognized as one of the top research scientists in the world. Having a specialty in cellular nutrition, Dr. Young has devoted his life to researching the true causes of “disease,” subsequently developing “The New Biology™” to help people balance their life. The pH Miracle series of books have sold over 10 million copies, have been translated into 29 languages and are gaining a widespread following internationally in over 159 countries.
Robert O. Young MSc, DSc, PhD, Naturopathic Practitioner

Please watch this every important documentary on VOTER FRAUD and HOW IT HAPPENED and WHAT WE CAN DO TO MAKE SURE EVERY LEGAL VOTE COUNTS!

Vote for the Person NOT the Party!
PSS Have YOU Been Bio-Chipped?
VAXXINE NANOTECH, TARGETING TECH, AI-TECHNO DOMINANCE OF HUMANITY
Informative and wide-ranging conversation with Dr. Robert Young, Rainetta Jones, and Melinda Czarnyszka Romanov examining the mRNA/liposome/graphene-laden vaccines now hooking people up to cloud computing and AI which is something people being targeted with these Smart City technologies as well as micro and nanotech implants and WBANs (wireless body area networks) and BCI (brain computer interface) implants have been reporting for a few decades now.
Covert aggressions by unlawful intelligence agencies, military divisions and biomedical research groups have led to people being extrajudicially implanted, tracked, monitored, and assaulted with electromagnetic and acoustic neuroweapons — a horrific scenario of targeting and persecution which is being hidden by “surveillance” and “mentally ill” paradigms emanating from local fusion centers and supported by corrupt media, law enforcement, and psychology/psychiatry professionals.
This is now being extended to millions through the vaccine nanotech. Also discussed was blood, the secrets of the blood and DNA, carrying our ancestors in us, and being our ancestors literally–as well as speculations on the targeting of soul and spirit as well as bloodline. Part 1 in a conversation to be continued below in Part 2!
Want to Learn More?

Special guest Dr. Robert Young makes his first appearance on the show to speak with Liberty Man about his microscopy and spectroscopy findings in the gene juice injections.

Dr. Young and Liberty Man talk about FDA corruption and share perspectives on the nanotechnology aspects of the shots, including topics such as RNA interference, toxicity, shedding, blood clots, and electromagnetic frequencies, and how all of this plays into the larger agenda of a digital ID social-credit prison system.
Related Video Lectures, Interviews & Peer-reviewed Scientific Papers



Extraordinary and riveting account of healing from terminal lung cancer and recovering from COVID symptoms on the pH Miracle Lifestyle healing program of alkaline diet, exercise, and healing foods from Dr. Robert Young, who describes the healing power of the right foods and actions to maintain the right pH balance in the interstitial fluids of the body, and encourages all to learn more about how disease is not transmitted by germs—as Virology would have it—but cultured in acidic environments, and how switching our diets and lifestyles from acidic to alkaline can transform our lives.


Peer-reviewed and Published June 20th, 2016 in the Journal of Vaccines and Vaccinations
[1] Young RO (2016) Second Thoughts about Viruses, Vaccines, and the HIV/AIDS Hypothesis – Part 1. Int J Vaccines Vaccin 2(3): 00032. DOI: 10.15406/ijvv.2016.02.00032

[2] Young RO (2016) Second Thoughts Concerning Viruses, Vaccines and the HIV/AIDS Hypothesis – Part 2. Int J Vaccines Vaccin 2(3): 00034. DOI: 10.15406/ijvv.2016.02.00034

[3] Young RO (2016) Second Thoughts Concerning Viruses, Vaccines and the HIV/AIDS Hypothesis – Part 3 HIV/AIDS and the Monomorphic Disease Model. Int J Vaccines Vaccin 2(3): 00035. DOI: 10.15406/ijvv.2016.02.00035



Published in the Early 90’s One Sickness, One Disease, One Treatment
One Sickness, One Disease, One Treatment First Edition Published in1996 – OUT of PRINT

“There is only one treatment, restore the alkaline design of the body fluids with an alkaline lifestyle and diet.” Dr. Robert O. Young – 1996

Robert O. Young MSc, DSc, PhD, Naturopathic Practitioner – www.drrobertyoung.com
Support the work, research and findings of Dr. Robert O. Young at: https://www.givesendgo.com/research

No donation is too small and will be appreciated and used for continuous research, publications and public education!
***
As a special thank you for your donation, I will answer one health or lifestyle related question.
After your donation, please email me at: phmiraclelife@gmail.com
Keep your question short and very specific. Please include your first and last name, phone number and email. Your question will be answered within 72 hours.
God bless YOU ALL!
Stay healthy and happy! www.givesendgo.com/researchwww.drrobertyoung.com/blog





