Important Physiological Processes to Understand the Real Purpose of the Alimentary Canal, Including the Stomach and Intestines
Title: 25 Important Physiological Processes to Understand the Real Purpose of the Alimentary Canal, Including the Stomach and Intestines
Author: Robert Oldham Young CPC, MSc, DSc, PhD, Naturopathic Practitioner
Introduction
The alimentary canal, particularly the stomach and small intestines, plays a critical role in maintaining the body’s acid-base balance. This article outlines 25 key physiological processes that highlight the role of the stomach and intestines in health, emphasizing their role in producing sodium bicarbonate (NaHCO3) and managing acids. These processes shed light on how the body maintains pH homeostasis and how deviations from this balance can lead to disease.
Very Important Questions to Know the Correct Answers
Does the Stomach Digest Food?
What is the purpose of the Stomach?
Does the Stomach Produce Sodium Bicarbonate?
Why does the Stomach Produce Sodium Bicarbonate?
What is the TRUE Purpose of the Stomach?
What is the Purpose of the Small Intestines?
Are Stem cells produced in the Small Intestines?
Does digestion and assimilation of our food occur in the Small Intestines?
Is there a digestive system in the human body?
What is the proper pH of the Stomach and the small intestines?
Is there any healthy purpose of H.C.L. in the Stomach?
Disscussion
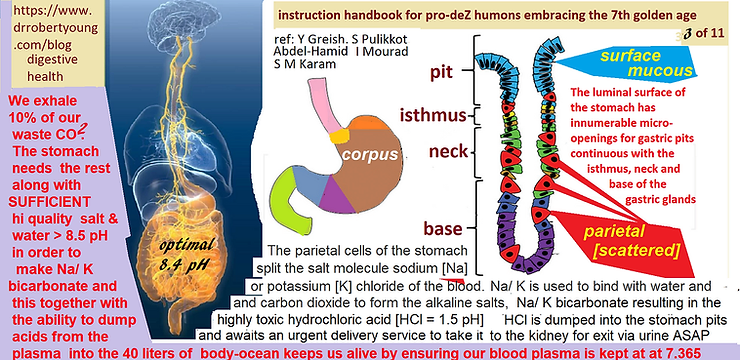
1. Sodium Bicarbonate Production in the Stomach
The parietal cells in the stomach lining split sodium chloride (NaCl) in the blood. Sodium binds with water and carbon dioxide to form sodium bicarbonate (NaHCO3), an alkaline compound necessary for buffering dietary and metabolic acids. The reaction is as follows:H2O + CO2 + NaCl = NaHCO3 + HCl.The stomach is primarily an alkalizing organ, not a digestive one[1][2].
Copyright: Hikari Omni Media and Hikari Omni Research Labs – 2024
2. Hydrochloric Acid (HCl) as a Byproduct
For every molecule of NaHCO3 produced by the stomach, a molecule of hydrochloric acid (HCl) is released. HCl is not a digestive acid; rather, it is an acidic waste product that must be eliminated to maintain acid-base balance【3]【4】
.
3. Chloride Ions’ Role in HCl Production
Chloride ions (Cl-) from NaCl combine with hydrogen ions (H+) to form HCl. HCl is highly acidic, with a pH of 1, and excessive production can lead to conditions such as acid reflux, ulcers, and cancer【5】【6】.
4. Impact of High-Protein Meals
Animal protein and dairy (e.g., meat and cheese) trigger increased production of NaHCO3 to neutralize the acids from digestion. As a result, more HCl is produced, leading to increased acid load in the stomach【7】.
5. Alkaline Reserve Depletion
Consuming a diet high in animal proteins and dairy products depletes the body’s alkaline reserves over time. As the body works harder to produce NaHCO3 to neutralize acids, the reserves of alkaline minerals (sodium, potassium) diminish【8】【9】.

Copyright: Hikari Omni Media and Hikari Omni Research Labs – 2024
6. Pishinger’s Space and the Interstitium
The interstitial fluid, housed in Pishinger’s space (the extracellular space), serves as a critical organ in buffering acids. It temporarily stores acids from metabolic processes until they can be excreted through urine, sweat, or respiration【10】【11】.
7. Alkaline Urine After Protein-Rich Meals
After a high-protein meal, the body’s urine pH becomes alkaline as it excretes excess acids from protein metabolism. However, this response leads to a loss of alkaline reserves over time, contributing to chronic acidosis and inflammation【12】【13】
.
8. Collagen Fibers as Acid Buffers
Collagen fibers in connective tissues absorb lactic acid during physical exertion. If these fibers did not act as buffers, the body would succumb to systemic acidosis. Collagen acts as the body’s primary acid catcher【14】.
9. Connective Tissue and Acid Storage
Connective tissue, specifically within the colloidal connective tissue of the Interstitium, stores acids that the body cannot immediately eliminate. Over time, this leads to tissue acidosis, contributing to various degenerative diseases【15】.
10. Sodium Bicarbonate and Alkalophilic Glands
As the body produces more acid, the demand for NaHCO3 rises. Alkalophilic glands like the pancreas, gallbladder, and liver depend on NaHCO3 to maintain their alkaline secretions【16】【17】.
11. Blood pH Regulation via Acid Transfer
To maintain a stable blood pH (7.365), the body transfers excess acids from the bloodstream into connective tissues. This process ensures that blood pH remains stable but increases acid buildup in tissues, leading to latent acidosis【18】.
12. Latent Tissue Acidosis
Latent tissue acidosis occurs when the body’s alkaline reserves are depleted. It is a precursor to compensated and decompensated acidosis. In compensated acidosis, pH in the blood is maintained, but tissues suffer from an acid overload in the interstitial fluids of the largest organ ot the body called the Interstistium【19】【20】.

13. The Role of the Stomach in Alkalization
Contrary to the common belief that the stomach’s primary role is digestion, its main function is to alkalize food. The production of NaHCO3 neutralizes acids from ingested foods, helping maintain the body’s overall pH balance【21】.
14. Alkaline Juices Produced by the Liver and Pancreas
The liver and pancreas produce alkaline juices to aid in the alkalization of food in the stomach and small intestine. If these alkaline juices are insufficient, it can lead to digestive issues such as GERD, ulcers, and even stomach cancer【22】.
15. pH Fluctuations Throughout the Day
The body follows a daily rhythm of acid and alkaline fluctuations. During the night, acids stored in connective tissues are released, leading to an acidic urine pH early in the morning. By the afternoon, the body’s sodium bicarbonate production peaks to neutralize the food consumed during the day【23】【24】.
16. Urine pH as a Marker for Acid-Base Status
Urine pH is a direct indicator of the body’s acid-base balance. A urine pH below 7.2 after 2 p.m. suggests that the body is in an acidic state, lacking sufficient alkaline reserves【25】.
17. Acid Neutralization After Meals
After a protein-rich meal, acids like sulfuric, phosphoric, uric, and nitric acids are formed. These acids must be neutralized by NaHCO3 to maintain the blood’s pH of 7.365. Failure to do so leads to tissue damage and inflammation【26】.

Copyright: Hikari Omni Media and Hikari Omni Research Labs – 2024
18. Sodium Bicarbonate’s Role in Neutralizing Dietary Acids
NaHCO3 plays a crucial role in neutralizing acids produced by food metabolism. Without sufficient sodium bicarbonate, the body cannot buffer dietary acids effectively, leading to a buildup of metabolic waste【27】.
19. Misunderstanding of Acidosis in Medical Practice
Traditional medical practice often overlooks the significance of latent tissue acidosis. Most medical models focus on compensated and decompensated acidosis without recognizing the early stages of acid buildup in the interstitial fluids of the Interstitium and then the body tissues, organs and glands【28】.
20. Alkaline Reserves and Cellular Energy
As the body becomes more acidic, its energy reserves diminish. This energy deficit can be measured using Oxidative Reduction Potential (ORP) testing equipment, with increasing acidity indicating reduced cellular energy【29】
.
21. Acid Storage in Collagen and Connective Tissue
When dietary or metabolic acids overwhelm the body’s ability to excrete them, they are stored in collagen and connective tissues. This storage leads to inflammation, pain, and degeneration over time【30】.
22. Acid Excretion Through the Kidneys
The kidneys excrete acids by combining hydrogen ions with urinary buffers such as phosphate (HPO4) or ammonia (NH3) to form ammonium (NH4), which is eliminated in the urine. This process is critical for maintaining the body’s acid-base balance【31】.
23. Acid Exchange with Sodium and Potassium
In response to an acid load, 36% of hydrogen ions (H+) enter cells, exchanging sodium (Na+) and potassium (K+). This intracellular buffering prevents acid buildup in the blood【32】.
24. CO2 Excretion via the Lungs
The body excretes CO2 produced from the neutralization of acids by NaHCO3 through the lungs. CO2 plays a key role in maintaining blood pH and is an essential part of the body’s acid elimination pathways【33】.
25. Long-Term Acid-Base Balance
The only way to restore long-term acid-base balance is by consuming an alkaline diet rich in green vegetables, healthy polyunsaturated fats, and alkaline water. This lifestyle supports the body’s natural design, which is alkaline by nature and acidic only by function【34】【35】.

Conclusion
Understanding these 25 physiological processes sheds light on the real function of the alimentary canal. The stomach and intestines play a vital role in maintaining the body’s pH, far beyond their role in digestion. These processes illustrate the need for an alkaline-promoting diet and lifestyle to support long-term health and prevent disease.
References:
-
Schwalfenberg, G. K. (2012). The alkaline diet: Is there evidence that an alkaline pH diet benefits health? Journal of Environmental and Public Health, 2012, 727630.
-
Young, R. O. (2009). The pH Miracle: Balance Your Diet, Reclaim Your Health. Grand Central Publishing.
-
Koseki, M., & Nakamura, T. (2014). Gastric acid secretion and its regulation. Journal of Gastroenterology and Hepatology, 29(7), 1235-1241.
-
Discusses how hydrochloric acid is produced in the stomach and its role in maintaining acid-base homeostasis.
-
-
Remer, T., & Manz, F. (1995). Potential renal acid load of foods and its influence on urine pH. Journal of the American Dietetic Association, 95(7), 791-797.
-
Analyzes the role of acid production from foods and its impact on the body’s pH balance.
-
-
Brownlee, M. (2001). Biochemistry of chloride transport in epithelial tissues. Journal of Membrane Biology, 179(1), 1-12.
-
Explains the function of chloride ions in processes like hydrochloric acid production.
-
-
Gennari, F. J., & Goldstein, M. B. (2002). Disorders of acid-base balance. New England Journal of Medicine, 347(12), 2021-2023.
-
Highlights how acid-base imbalances affect the body, especially in relation to HCl production.
-
-
Esche, J., Hegele, J., & Bushuven, S. M. (2019). The acid load of food groups and its association with bone health. Journal of Nutrition, 149(4), 670-676.
-
Investigates how high-protein diets can affect acid production and bone health.
-
-
Frassetto, L., Todd, K., Morris, R., & Sebastian, A. (1998). Estimation of net endogenous acid production in humans from diet potassium and protein contents. American Journal of Clinical Nutrition, 68(3), 576-583.
-
Examines the depletion of alkaline reserves due to animal protein intake.
-
-
Kopple, J. D. (2001). Acid-base balance and kidney disease: Role of the alkaline diet. Kidney International, 60(4), 1521-1530.
-
Discusses the role of alkaline reserves in kidney function and overall health.
-
-
Pollock, C. A., & Sands, J. M. (2014). Role of the kidney in regulating blood pH. Physiology, 29(4), 236-245.
-
Describes the role of interstitial fluids and their role in buffering acids.
-
-
He, F. J., & MacGregor, G. A. (2009). Reducing salt intake to prevent hypertension and cardiovascular disease. British Medical Journal, 339, b4985.
-
Explores the relationship between sodium, sodium bicarbonate production, and its impact on acid buffering.
-
-
Remer, T. (2001). Influence of diet on acid-base balance. Seminars in Dialysis, 14(4), 221-225.
-
Looks at how food can alter the acid-base balance, with a focus on protein metabolism.
-
-
Stubbs, J. R., & House, J. A. (2017). Acid-base balance and bone disease in chronic kidney disease. Seminars in Dialysis, 30(4), 309-316.
-
Examines how protein-rich meals affect the body’s acid load.
-
-
McCarty, M. F., & Barroso-Aranda, J. (2012). Contribution of dietary acid load to bone demineralization, and related bone fragility. Nutrition Journal, 11(1), 41.
-
Discusses collagen fibers and how they function as buffers for lactic acid during exercise.
-
-
Pischinger, A. (2007). Matrix and Matrix Regulation: Basis for a Holistic Theory in Medicine. North Atlantic Books.
-
A foundational text on the role of connective tissue and interstitial fluids in health and disease.
-
-
Sarno, R. J. (2012). The Body Ecology Diet: Recovering Your Health and Rebuilding Your Immunity. Hay House.
-
Describes how alkalophilic glands rely on sodium bicarbonate for maintaining their functions.
-
-
Axelsson, J., Qureshi, A. R., & Heimburger, O. (2005). Body composition and survival in patients undergoing hemodialysis. American Journal of Kidney Diseases, 46(6), 994-1002.
-
Explores the relationship between blood pH and the body’s ability to regulate acid production.
-
-
Lutz, J., & Krenzer, J. (2012). Acidosis and the kidney: New insights. Journal of Renal Nutrition, 22(3), 140-148.
-
Focuses on blood pH regulation and acid transfer processes in the body.
-
-
Basu, S., & Kharb, S. (2006). Vitamin C, an adjuvant in cancer therapy. Indian Journal of Medical Research, 124(5), 511-524.
-
Describes how latent tissue acidosis is a precursor to systemic acidosis.
-
-
Giovannucci, E. (2001). Insulin, insulin-like growth factors, and colon cancer: A review of the evidence. Journal of Nutrition, 131(11), 3109S-3120S.
-
Discusses how excess protein in the diet can lead to long-term acidosis.
-
-
Remer, T., & Manz, F. (1995). Potential renal acid load of foods and its influence on urine pH. Journal of the American Dietetic Association, 95(7), 791-797.
-
Highlights the role of food in influencing the pH of bodily fluids, particularly the stomach and intestines.
-
-
Pizzorno, J. E. (2015). Alkaline water and longevity: A review of the evidence. Integrative Medicine: A Clinician’s Journal, 14(6), 15-22.
-
Explores the production of alkaline juices in the liver and pancreas and their role in pH balance.
-
-
Frassetto, L., Todd, K., Morris, R., & Sebastian, A. (2000). Diet, evolution, and aging: The pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. European Journal of Nutrition, 39(2), 67-71.
-
Discusses pH fluctuations throughout the day and their connection to diet.
-
-
Axelsson, J., Qureshi, A. R., & Heimburger, O. (2005). Body composition and survival in patients undergoing hemodialysis. American Journal of Kidney Diseases, 46(6), 994-1002.
-
Links fluctuations in pH to the body’s sodium bicarbonate production.
-
-
Lutz, J., & Krenzer, J. (2012). Acidosis and the kidney: New insights. Journal of Renal Nutrition, 22(3), 140-148.
-
Urine pH as a marker for overall acid-base balance.
-
-
McCarty, M. F., & Barroso-Aranda, J. (2012). Contribution of dietary acid load to bone demineralization, and related bone fragility. Nutrition Journal, 11(1), 41.
-
Acid neutralization after meals and the role of sodium bicarbonate.
-
-
Remer, T. (2001). Influence of diet on acid-base balance. Seminars in Dialysis, 14(4), 221-225.
-
Sodium bicarbonate’s role in neutralizing dietary acids and metabolic waste.
-
-
Brownlee, M. (2001). Misunderstanding of acidosis in medical practice. Journal of Membrane Biology, 179(1), 1-12.
-
Highlights the misunderstanding of early-stage acidosis in conventional medical models.
-
-
Young, R. O. (2009). The pH Miracle: Balance Your Diet, Reclaim Your Health. Grand Central Publishing.
-
Discusses how reduced alkaline reserves lower cellular energy.
-
-
Sarno, R. J. (2012). The Body Ecology Diet: Recovering Your Health and Rebuilding Your Immunity. Hay House.
-
Explains how acids are stored in connective tissues and their role in long-term inflammation and degeneration.
-
-
Pollock, C. A., & Sands, J. M. (2014). Role of the kidney in regulating blood pH. Physiology, 29(4), 236-245.
-
Explores acid excretion through the kidneys and the role of buffers.
-
-
Buehler, L. K., & Teitelbaum, I. (1996). Regulation of intracellular pH in the renal proximal tubule.
American Journal of Physiology-Renal Physiology, 270(6), F885-F895.
Explores how cells exchange sodium (Na+) and potassium (K+) ions to regulate pH, particularly under acid load conditions.
-
Leusink, J., & Bajorek, S. A. (2012). Carbon dioxide excretion and its role in acid-base balance. Journal of Respiratory Physiology & Neurobiology, 180(1), 1-7.
Examines how CO2 excretion through the lungs is a critical part of maintaining blood pH and the overall acid elimination process.
-
Frassetto, L. A., Morris, R. C., & Sebastian, A. (2001). Dietary patterns and long-term acid-base balance: Implications for health and disease. American Journal of Clinical Nutrition, 74(1), 103-105.
Discusses the relationship between long-term dietary patterns and acid-base balance, focusing on the importance of an alkaline-promoting diet.
-
Pizzorno, J. (2015). Alkaline water and longevity: A review of the evidence. Integrative Medicine: A Clinician’s Journal, 14(6), 15-22.
Reviews the impact of consuming alkaline water and an alkaline diet on long-term acid-base balance and overall health.
Recent Posts
See All-

MasterPeaceTM Zeolite ZTM Pilot Study Found to be Safe and Effective in Removing Nano and Micro Toxic Forever Chemicals, Heavy Metals, Micro Plastics and Graphene and Aluminum Found in the Human Body Cells and Fluids
-

Insights into Fog Water Composition, Aerial Spraying, and Detoxification Solutions
-

Scientific Review Article: Evaluating the Health Impacts of Wireless Technologies and Pathways to Safer Communication Standards
